The Central Drugs Standard Control Organisation (CDSCO) has issued a strong directive to all State and UT Drug Controllers to immediately stop the manufacture, sale, and distribution of 35 unapproved Fixed Dose Combinations (FDCs). These drugs were being sold without proper approval, posing a serious risk to public health.
The circular, dated April 2025, emphasizes that these combinations violate the New Drugs and Clinical Trials (NDCT) Rules, 2019, and the Drugs & Cosmetics Act, 1940.
❌ What Are Fixed Dose Combinations (FDCs)?
FDCs are medicines that combine two or more active ingredients in a fixed ratio in a single dosage form. While many FDCs are safe and effective, others can be dangerous if not scientifically evaluated for safety, efficacy, and drug interactions.
🚫 Why Were These 35 FDCs Banned?
According to CDSCO, many of these FDCs:
- Were approved by State Licensing Authorities (SLAs) without central clearance
- Had no proper evaluation for safety and efficacy
- Posed risks of side effects, drug interactions, or overmedication
- Were marketed in violation of national drug laws
🔍 Common Types of Drugs in the Banned List:
The banned FDCs include combinations of:
- Antidiabetics (e.g., Metformin + Glimepiride + Dapagliflozin)
- Antibiotics (e.g., Cefixime + Ofloxacin + Lactic acid bacillus)
- Painkillers and Cold Medications (e.g., Dextromethorphan + Phenylephrine + Diphenhydramine)
- Multivitamins and Supplements
- Antifungal and Steroid Creams
- Psychiatric Drugs (e.g., Sertraline + Clonazepam)
- Injections (e.g., Meropenem + Avibactam)
🧪 What Are the Dangers?
Using unapproved combinations without scientific validation can lead to:
- Adverse drug reactions
- Toxic effects
- Reduced treatment effectiveness
- Misuse or overuse of antibiotics contributes to antimicrobial resistance
🛑 CDSCO’s Directive to State Authorities:
- Cancel the licenses of all banned FDCs immediately
- Reassess approval processes for FDCs
- Investigate and report cases to the CDSCO
- Ensure strict compliance with NDCT Rules and the Drugs & Cosmetics Act
- Treat this issue as urgent and critical
🧾 Full List of 35 Banned FDCs:

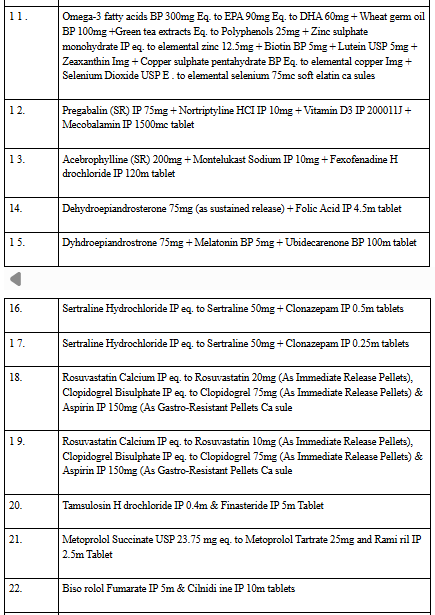
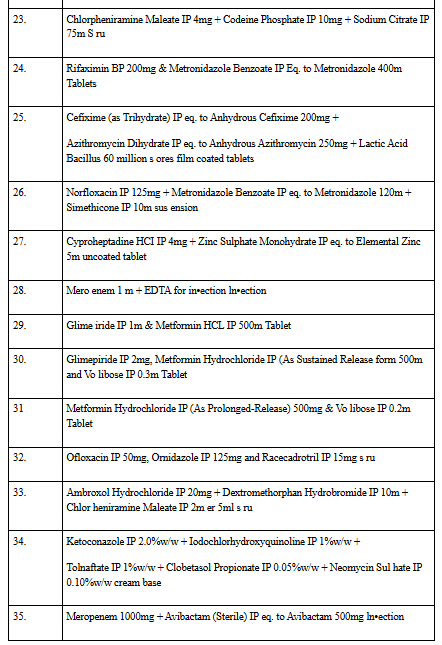
📢 Call to Action for Doctors:
🩺 Check your inventory: Ensure none of these FDCs are being prescribed or stocked
📞 Inform patients who may be taking these combinations to consult for safe alternatives
📚 Stay updated with CDSCO notices and NDCT regulations
🧑⚕️ Report any adverse events linked to unapproved FDCs to the authorities
🔗 Work closely with pharmacists and drug suppliers to ensure compliance and safety
Let’s work together to ensure that only scientifically approved, safe, and effective medications are used in clinical practice.
To register for our next masterclass, please click here https://linktr.ee/docpreneur




